- Research
- Open access
- Published:
Patient-physician communication of health and risk information in the management of cardiovascular diseases and diabetes: a systematic scoping review
BMC Medicine volume 23, Article number: 96 (2025)
Abstract
Background
The communication of health and risk information is an integral part of patient-physician interaction. Effective communication of risk information for cardiovascular diseases and diabetes has been shown to improve medication adherence, increase physical activity levels, and improve dietary control. Patients who understand their risk profile are better able to work towards modifying their lifestyle behaviours as part of a shared decision-making process with physicians. This scoping review examines the evidence on patient-physician risk communication strategies, approaches and interventions for CVDs and diabetes management in primary care and secondary outpatient settings.
Methods
A comprehensive database search for quantitative and qualitative studies was conducted in PubMed, Embase, Web of Science, Scopus, CINAHL, PsycINFO, and Cochrane Library from 1st January 2000 to 3rd October 2023. Two reviewers independently performed the screening of articles. Studies that report on patient-physician risk communication processes were included. Data were extracted and analysed using descriptive summaries and narrative synthesis. Results are reported in accordance with PRISMA-ScR guidelines. Included articles were appraised for quality following JBI critical appraisal and MMAT tools.
Results
A total of 8378 articles published between 1st Jan 2000 to 3rd October 2023 were screened. After a full-text review of 88 articles, a total of 30 articles, consisting of 15 qualitative, 14 quantitative and 1 mixed method studies were included. Common areas of inquiry among articles include: (1) understanding and recalling risk information, (2) strategies and approaches used by physicians to communicate risk, and (3) interventions to improve the communication of risk. Studies reveal how physicians use a range of strategies, approaches and interventions to discuss risk with patients. We present and discuss each theme narratively in detail.
Conclusions
There is a critical need for further research into risk communication strategies for cardiovascular diseases (CVDs) and diabetes, with a focus on developing targeted approaches that enhance patients' understanding of their risk profiles. Evidence-based guidelines should assist healthcare professionals improve risk communication within clinical settings, with the goal of facilitating patient comprehension of health risks that can sustain lifestyle changes. Misalignment in communication may lead to dissatisfaction and confusion, which may impede the effective management of chronic conditions.
Background
Patient-physician communication is an integral component of clinical care in the management of chronic diseases such as cardiovascular diseases (CVDs) and diabetes. Not only do physicians need to establish rapport and trust with patients during consultations to facilitate the exchange of personal health information, patients have to also voluntarily provide details about their medication intake and lifestyle behaviour so that physicians can provide an accurate prognosis for each patient. Guided by the natural history progression, present health status, and specific risk factors or symptoms exhibited by each patient during clinical encounters, physicians aim to accurately evaluate and recommend suitable treatment plans for patients to follow.
For chronic conditions such as cardiovascular diseases and diabetes which are heterogeneous and multi-factorial in aetiology [1, 2], physicians need to be cognizant of individual as well as general factors while assessing a patient. Individual level factors such as age, gender, the specific stage or duration of the condition, an outline of co-morbidities, present physiological ability, and the social background of each patient are evaluated in tandem with standardised indicators representing the persistence or progression of chronic conditions. These standardised indicators are clinical guidelines recommended by professional bodies [3,4,5] and are reference points to determine if treatment to target thresholds are met and whether a patient’s overall condition is adequately managed [6].
Whilst physicians try to present a realistic description of risks appropriate for each patient, actual communication processes involve reaching out, connecting with, and empathising with a patient’s circumstances and social background [7]. The negative consequences and dangers of disease risks have to be tempered by understanding each person’s unique psychosocial background and personal challenges a patient is going through [8]. By showing compassion, care, and listening actively, patients develop greater trust and will be more activated to take charge of their own health [9, 10]. Physicians in turn may be better able to guide patients to control their health indicators such as haemoglobin A1c, blood glucose, and cholesterol levels [11].
Numerous factors can significantly affect the overall quality of communication that impede the communication of health and risk information between physicians and patients. These factors include the communicative style of physicians [12], tone, nonverbal gestures [13], and ethnic and cultural concordance between patients and physicians [14, 15]. Contextual factors such as literacy or education levels [16], language ability and comprehension [17], numerical ability of patients [18], ethnicity or race factors [19], financial costs concerns [20], and other emotive considerations [21], can also mediate each level of discussion. From a provider’s perspective, physicians may find it challenging to balance empathy and compassion in the context of systemic pressures, where workload and time constraints can inhibit optimal conversations that impact health outcomes [22,23,24].
Despite the clear implications of effective risk communication and the key role it plays in chronic disease management, dyadic patient-physician risk communication remains sub-optimal and faces considerable challenges in practice [25]. One recent systematic review that examined risk communication strategies focused only on general population studies and population groups without known CVDs [26], while another review examined only qualitative and mixed methods studies, looking at how patients with coronary heart disease experience risk communication in the context of health education [27]. One systematic review focused only on the format and type of risk presentation used in intervention studies, looking at how format affects a patient’s understanding, affect and intention to change [28]. There is a need to understand how risk is communicated in primary or outpatient settings since physicians are often the primary gatekeeper or main point of contact for patients who are diagnosed with chronic conditions that have not progressed to severe stages.
Aims and rationale
The purpose of this systematic scoping review is to scope the existing literature on patient-physician risk communication related to CVDs and diabetes management in the primary care or secondary outpatient setting. The PICO framework was used to guide the formulation of research questions for this scoping review:
Patient or problem
Participants with cardiovascular diseases or type 1 or 2 diabetes, aged 18 years old and above.
Intervention or exposure
Interaction with a physician/clinician(s) pertaining to a patient’s cardiovascular diseases or Diabetes.
Comparison
Not applicable.
Outcome measures
-
Studies or articles that report about communication between physicians and patients with cardiovascular conditions or Diabetes in the primary or secondary care setting.
-
Quantitative studies may be observational or be part of an intervention study. Observational studies should include studies that examine processes of risk communication and any health or behavioural effects that arise over the short to long term because of risk factors.
-
Interventions that have the intention to improve risk communication practices to shape or influence health and behavioural outcomes will be included in this study.
-
Quantitative studies utilising surveys asking about patient-physician or clinician interactions, with risk communication components will be included.
-
Outcome results will be reported for quantitative studies relevant to the communication of risk.
-
All qualitative study types and methods will be included in this review, including studies using narrative inquiry, ethnographic, participant observation, phenomenological, grounded theory, and/or other qualitative methods.
We include both CVD and diabetes since there are clear pathophysiological pathways describing diabetes as a risk factor for CVDs [29, 30], and comprehensive risk management is needed for patients with diabetes to reduce risk levels through pharmacological and lifestyle approaches [31]. Although the focus of this review is on the communication of risks, we examine both health and risk information and treat both as closely interconnected given how risk is often embedded within larger conversations about health and health information within clinical settings [32].
The aim of this review is to map the available literature, and address the following study questions:
-
1.
What aspects of risk communication and understanding of risk are reported in the primary care or outpatient setting?
-
2.
What communication tools are used to facilitate patient-physician risk communication?
-
3.
What components of interventions are applied in studies to improve patient-physician risk communication in the clinical setting?
-
4.
What are the gaps in the literature related to patient-physician risk communication?
Methods
Protocol registration and guidelines
We follow Arksey and O’malley’s (2005) [33] list of stages as an overarching guide in conducting this scoping review. The Joanna Briggs Institute (JBI) guidance was used to develop a scoping review protocol, which has been registered on the Open Science Framework platform (https://doiorg.publicaciones.saludcastillayleon.es/https://doiorg.publicaciones.saludcastillayleon.es/10.17605/OSF.IO/CQFD5). We use the PICO guideline incorporated in the protocol as a framework to help structure questions in planning for this review, and in starting out with a list of search terms [34]. In reporting the findings of this review, we follow the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines (Page et al. 2020, Tricco et al. 2018) (Additional File 1) [35, 36]. During the review of full-text articles, we adjusted the list of aims in the protocol to align more closely with the list of articles generated, so that the scope of the questions centre on the methods, tools, and interventions used to improve risk communication.
Publication type and study design inclusion
This scoping review considers experimental and quasi-experimental study designs including randomised and non-randomised controlled trials, before and after studies, and interrupted time-series studies. Analytical observational studies were considered for screening, including prospective cohort, case–control, analytical cross-sectional, and retrospective cohort studies. All qualitative and mixed methods study types are considered for this review. Protocols, case series, case studies, or case reports were excluded from this review. Editorial, opinion, commentary, review papers, professional guidelines, conference abstracts or reports, and letters were also excluded. Non-English studies were excluded. During the screening process, we decided to include descriptive cross-sectional studies if they inform the study aims.
Database search and information sources
Abstract and full text in English published between 1st January 2000 to 3rd October 2023 were searched from 7 databases, including PubMed, Embase, Web of Science, Scopus, CINAHL, PsycINFO, and Cochrane Library, with the help of a medical librarian (YM). The librarian tailored the final search strategy for each database. The search terms were refined collaboratively between a reviewer (AC) and the librarian over a few sessions and discussed with a second reviewer (WT) to check that the results were relevant. An initial list of search terms was first implemented on Pubmed, followed by a review of the first 250 results by the first reviewer (AC) to check if the range of articles in the search results was acceptable. The librarian then provided additional suggestions to improve the search terms, such as adding a series of MeSH terms so that all CVD and diabetes articles would be adequately included. It was decided between the reviewers to start the search from 2000 to scope for recent articles on patient-physician health and risk communication.
Search terms were grouped into 2 broad categories. The first set of terms covers broadly the span of conditions related to CVD or diabetes, such as “cardiovascular diseases” or “diabetes milletus” using MeSH terms, which includes all associated conditions that fall under each MeSH category. The first set of terms were then combined with a Boolean AND with the second set of terms focusing primarily on patient-physician communication or risk communication, using terms such as “risk communication*” or “patient-provider engagement”. Terms were truncated to include variations to broaden search terms. The final search strategy and full list of search terms applied to all databases can be found in Additional file 2.
Screening and study selection
After the initial implementation of the search terms across databases and duplicates removed by the medical librarian, two reviewers (AC and WT) screened article titles and abstracts independently using Rayyan [37]. Reviewers met regularly at monthly intervals to discuss and resolve discordant articles that were included or excluded, and to ensure consistency in articles screened. Any disagreement was resolved through discussion and by consensus between reviewers. One key screening decision made during multiple meetings was to exclude articles that did not specifically involve a patient-physician interaction or explicitly report a patient-physician communication process, even if risk perception or risk communication is reported as part of the study. Another decision made after discussion was to include studies on risk communication even if participants are recollecting about a patient-physician encounter or who was interviewed/surveyed post-visit that has not necessarily taken place within a clinical setting.
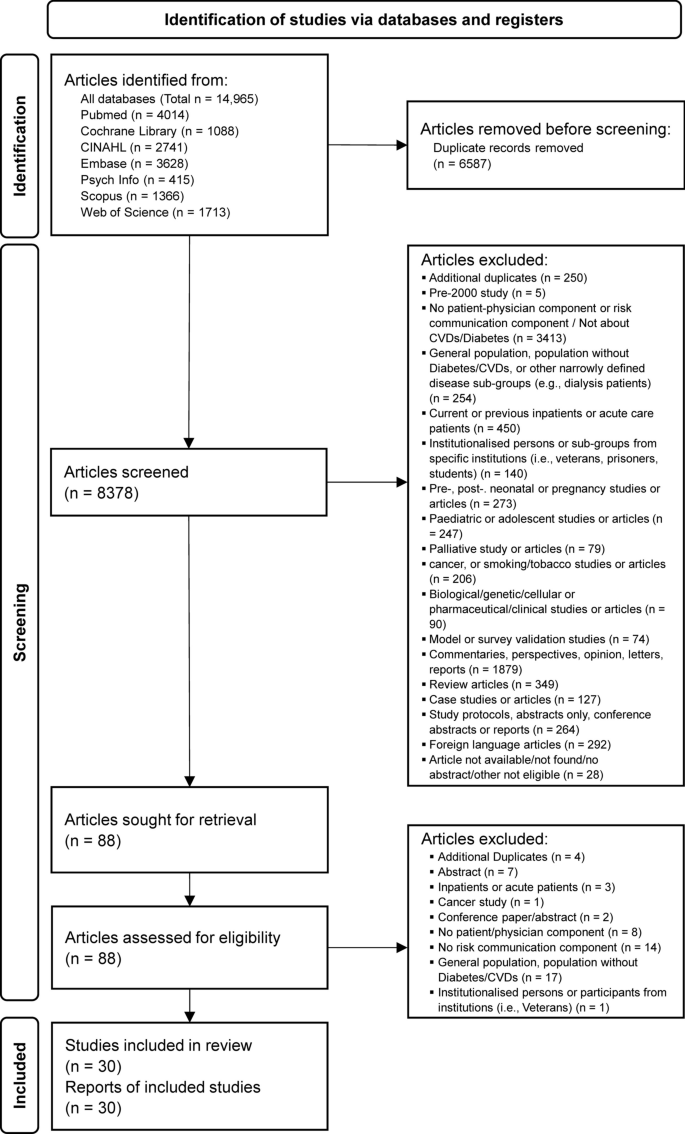
A total of 8378 articles were screened. After a full-text review of 88 articles, a total of 30 articles, consisting of 15 qualitative, 14 quantitative and 1 mixed method studies were included. To the best of our knowledge, this is the first systematic scoping review to focus on patient-physician risk communication in the primary or outpatient care setting for both CVDs and diabetes using the search terms generated.
Eligibility criteria
Inclusion criteria
Included studies are quantitative or qualitative studies that report on the communication of health and risk information between physicians and patients with cardiovascular diseases or diabetes in the primary or outpatient care setting. Quantitative studies may be observational or intervention studies. Observational studies include studies that examine processes or aspects of risk communication and any health and behavioural effects that arise because of risk communication processes. Interventions that have the intention to improve risk communication practices that shape or influence behavioural outcomes are included for consideration in this study. All qualitative study types utilising different qualitative methodologies are included in this review for screening.
Definition of primary care and outpatient settings
To ensure consistency during screening, we refer to primary care as used by the American Association of Family Physicians (AAFP), as the “…provision of integrated, accessible health care services by physicians and their health care teams who are accountable for addressing a large majority of personal health care needs…practicing in the context of family and community” [38]. Following terminology from the WHO Global Health Observatory, outpatient settings is defined as the site “…of health service, between a non-hospitalized individual and a health worker responsible for the evaluation, diagnosis, treatment, or referral of that person in that encounter.” [39]. An outpatient visit is a patient who is in “…contact with a health professional…and is not admitted to any health care facility and does not occupy a hospital bed for any length of time.” [40].
Excluded studies
We excluded studies where the intended aim of the study or study population is not about CVD or diabetes, or general population studies where populations with CVD or diabetes are not explicitly demarcated. Groups that have an aggregate risk profile that can vary markedly and distinctly for specific sub-groups were excluded, including paediatric, adolescent, pregnancy, natal, postpartum, cancer, dialysis, smoking and tobacco studies, or studies involving institutionalised or formerly institutionalised groups such as veterans, military personnel, prisoners, or students, the rationale being that the emphasis and context of risk communication may differ substantially for these specific groups. We excluded studies conducted in inpatient, emergency department or palliative care settings that typically admit acute patients, whose aggregate risk tends to be higher than patients who tend to visit primary or outpatient care settings. Validation studies of surveys, modelling studies, animal, microbiological, cellular, or genetic studies that don’t typically involve patient-physician communication were excluded. After some deliberation, the study team decided to exclude systematic or other review articles that may use a separate set of selection criteria in the selection of studies. A summary of included and excluded studies can be found in Table 1.
Data extraction
Data extraction was conducted by a reviewer (AC), who read through all included articles thoroughly, based on a data extraction template that was continuously revised and updated accordingly from the protocol version as more included studies were reviewed. Given the heterogeneous nature of studies, we used a framework to categorise the extracted literature following the study aims (Fig. 1). First, key descriptive details from all articles were extracted by a reviewer (AC) to an overview data table that reports the following attributes: author, year, country, study design/data collection approach, sample size, study population brief characteristics, study aims/intervention or approach, and key findings.
The reviewer then manually analysed the overview data table to determine which articles can be categorically grouped with one another. Articles were grouped into the following thematic areas, each of which corresponded directly to the study aims: (1) understanding risk in the context of patient-physician communication (study aim 1), (2) strategies and approaches used by physicians in patient-physician risk communication (study aim 1), and (3) interventions to improve the communication of risk information (study aims 2 and 3). Details emergent from each article are then recorded to further populate data tables for this study (Tables 2, 3, 4 and 5). Theme (1) focuses mainly on patients' perspectives, (2) focuses mainly on the physicians' perspectives, while (3) summarises the list of intervention components that have been used to enhance risk communication.
To ensure consistency in data extraction and to handle heterogeneous reporting of results, definitions of what items to extract were made as clear as possible from the start of this review. For instance, both samples at allocation and data analysed were extracted consistently from randomised trials unless not available. For qualitative studies, strategies and approaches described have to be directly relevant to risk communication between patients and physicians before extraction. Data was extracted mainly from the results section for qualitative and mixed methods studies, and from the results section and data tables for quantitative studies. Interventions for randomised or controlled trials were typically extracted from the methods section of relevant articles. A second (WT) and third (SA) reviewer subsequently checked and validated the data extracted independently. Any differences in the data extracted were resolved through discussion and consensus (Table 1).
Quality appraisal
A quality appraisal was conducted to check for the quality of the articles selected for this systematic scoping review. For qualitative articles included, we refer to the Consolidated criteria for reporting qualitative research (COREQ) guidelines [71] to appraise the articles. A reviewer (AC) first assessed all qualitative articles to check if the articles met the list of criteria provided, a second reviewer (WT) subsequently validated the accuracy of the appraisal conducted by the first reviewer, based on a selected number of articles. Any differences were resolved through discussion by both the first and second reviewers.
For quantitative studies, the JBI critical appraisal tool for RCTs [72], quasi-experimental [73], and cross-sectional studies [74] were used to appraise included studies. Quantitative articles were first reviewed by a reviewer (SA), who is a senior statistician, followed by a second reviewer (AC), who assessed the quality of selected articles independently. Differences were resolved through discussion. The JBI appraisal tool was selected to minimise the use of multiple different appraisal tools for quantitative studies. The only mixed methods study included in this review was assessed by a reviewer using the mixed methods appraisal tool [75]. A summary of all the studies assessed and a list of criteria checked can be found in Additional file 3.
Results
A total of 8378 article titles and abstracts were screened from all databases, of which 8348 were excluded based on the eligibility criteria after consensus was reached by the reviewers (AC, WT). Eighty-eight articles were sought for retrieval for full text to be read and reviewed independently by both reviewers, after which a further 58 articles were excluded. A total of 30 articles were included for data extraction for this review (Fig. 2).
Description of included studies on patient-physician risk communication
Studies identified were heterogenous in scope and focus. Of the 30 of articles included, 22 articles are related to CVDs (n = 22/30, 73.3%), comprising 13 qualitative (n = 13/22, 59.1%), 9 quantitative studies (n = 9/22, 40.9%), and 1 mixed methods study (n = 1/22, 4.5%). Out of 13 qualitative studies, 10 used a semi-structured interview approach to data collection (n = 10/13, 76.9%), with 1 study using a qualitative descriptive approach (n = 1/13, 7.7%), 1 that conducted focus group discussions (n = 1/13, 7.7%), and 1 using both participant observation and interviews (n = 1/13, 7.7%). Out of 9 quantitative studies related to CVDs, 7 were randomised controlled or controlled trials (n = 7/9, 77.8%), 1 an interrupted time-series study (n = 1/9, 11.1%), and 1 a cross-sectional study (n = 1/9, 11.1%). 7 out of 30 articles included in this review are related to diabetes (n = 7/30, 26.7%), comprising a total of 2 qualitative (n = 2/7, 28.6%) and 5 quantitative studies (n = 5/7, 71.4%). Out of the 2 qualitative studies, 1 used a semi-structured interview (n = 1/2, 50.0%), and 1 a focus group discussion approach (n = 1/2, 50.0%). For 5 quantitative studies related to diabetes, 3 studies were randomised controlled trials (n = 3/5, 60.0%) and 2 studies were cross-sectional (n = 2/5, 40.0%).
Studies selected were conducted in or referred to primary or secondary care settings. Of 22 articles on CVD, 17 studies referred to primary care settings (n = 17/22, 77.3%), while 3 occurred in secondary care settings (n = 3/22, 13.6%), and 3 in both primary and secondary care settings (n = 3/22%). Primary care settings were mainly GPs (n = 11/17, 64.7%), family practices (n = 3/17, 17.6%), and community health centres (n = 1/17, %). 2 studies did not state specifically the actual primary care type (n = 2/17, 11.8%). Secondary care settings were mainly atrial fibrillation or transient ischemic attack clinics (n = 3/17, 17.6%). 3 studies that included participants from both primary and secondary care settings did not specifically state the exact venue of research conducted. Only 1 mentioned primary care setting as GP sites. Out of 7 articles on diabetes, 3 studies referred to primary care settings (n = 3/7, 42.9%), and 4 to secondary care settings (n = 4/7, 57.1%). Primary care settings were GPs, community health service centres, and 1 which was not stated. Secondary care settings were a teaching hospital, university clinic, a surgery practice, and a diabetes centre. A summary of key characteristics of included studies can be found in Table 2.
Most studies on patient-physician risk communication were conducted and concentrated in several high-income countries (HICs) in Europe, North America, and Oceania, such as the UK (n = 7/30, 23.3%), Germany (n = 4/30, 13.3%), Netherlands (n = 3/30, 10.0%), Denmark (n = 1/30, 3.3%), Sweden (n = 1/30, 3.3%), France (n = 1/30, 3.3%) in Europe; USA (n = 7/30, 23.3%), Canada (n = 1/30, 3.3%) in North America, and 1 each in Australia and New Zealand, respectively. Only 1 (1/30, 3.3%) [70] study was conducted in an upper middle-income country (UMICs) (China), and only 2 (2/30, 6.6%) [60, 63] conducted in 2 lower middle-income countries (LMICs) (Egypt and Indonesia), suggesting a paucity of research from non-HIC countries. There were no studies from countries in Africa, South America or other parts of Asia other than China. A summary of countries where studies are conducted can be found in Table 3.
Understanding risk in the context of patient-physician communication
CVD-related risk information
Fifteen articles, consisting of 8 qualitative [45, 47, 49, 50, 52, 53, 56, 59], 6 quantitative studies [41, 42, 51, 57, 60, 62] and 1 mixed methods study [54] focused on different aspects of patient-physician risk communication. We summarise and cluster the articles into 3 thematic areas emergent from articles on CVDs: (1) understanding and recalling risk information in the context of patient-physician communication, (2) risk formats and their effects on the risk communication process, and (3) perception of risk information over time.
Understanding and recalling risk information
Many patients tend to perceive risk in binary terms, such as whether they were ‘at risk’ or ‘not’ [52], or understood future risks in generic, non-numeric terms, even if numeric values were often used to discuss weight, blood pressure and medication dosage [56]. A qualitative study of patients with high 10-year CVD risk in the UK found that most patients do not remember receiving explanations about their CVD risk score or what their scores mean [53]. As such, most healthcare professionals tend to explain risk narratively rather than describe risk in percentage terms to patients [53]. In a study conducted in Vancouver, Canada, physicians describe how atrial fibrillation (AF) patients tend to overestimate their bleeding risk regarding anti-coagulants and have difficulty weighing risk against benefits [59]. In response to a lack of interest or inability of patients to understand their own risk, physicians communicate an individual’s risk of stroke to a patient less often unless there is a need to do so, such as when patients show resistance towards medications, if there is an unjustified fear of bleeding, or where there is poor understanding towards how medications can reduce risk [59].
For patients with asymptomatic conditions, a common problem is recognising and acknowledging risks that may not be apparent due to a lack of symptoms. A qualitative study to understand the experience of transient ischaemic attack (TIA) patients during consultation sessions found that prior knowledge and health beliefs influence actions taken by patients and that a lack of symptoms leads to less recognition of risks [47]. Many patients, such as those with high cholesterol who do not have manifest symptoms, find their risks unpredictable, unstable and abstract. These patients also have a poor understanding of CVD risk factors and do not perceive hypercholesterolemia to be a risk factor for CVDs [49]. Physicians of AF patients find it worrying that patients often associate symptom severity with the risk of stroke, who believe correspondingly that having a lack of symptoms implies not being at risk [59].
One intervention study using probabilistic scenarios conducted with GPs, healthcare assistants, and laypeople to test the level of minimum absolute risk required for participants to justify prescribing a hypothetical tablet able to prevent heart attack over 5 years, found that most participants think it makes no difference if a drug is consumed over 10 instead or 5 years, even if the benefit was greater over a longer time period, suggesting challenges in risk estimation even for healthcare professionals [50]. An intervention study that aims to facilitate the communication of CVD risks between patients and physicians by providing patients with a tablet containing a series of educational modules that patients have to watch prior to consultation sessions, found that having a sequence of educational intervention makes it easier for patients to speak to their physicians and have a better understanding of why controlling CVD risk factors is important [57].
Different risk formats and its effect on the risk communication process
Multiple studies focused specifically on risk formats and how they shape the risk communication process. 1 qualitative study that interviewed GPs in New South Wales, Australia, suggests how pragmatic considerations can affect how physicians choose to convey risk [45]. The study finds that physicians prefer using qualitative formats for communicating risk to patients who have lower numeric literacy and who are of lower risk, given how discussion of numbers with patients may take up a substantial amount of time. Absolute, relative, and risk displayed in a frequency format, were preferred formats used by GPs to convey information to patients who are at high risk or who had a poorly managed CVD condition. For patients, there was the perception that providing both absolute and relative risk calculations may be unnecessary and confusing since limited information about ways to reduce risk was already received by patients in the first place. Some patients had strong objections to the word ‘absolute’, which was seen as ambiguous and seemed to convey a risk score that was ‘conclusive’ or ‘definitive’ [54].
There was consensus among patients that risk was generally difficult to understand. In one mixed methods study conducted in the UK to evaluate the use of the JBS2 risk calculator and chart within GP settings, patients do not recall seeing a risk assessment tool used, although they agree that the use of tools can increase confidence in risk assessment and aid patients in understanding risks [54]. Patients prefer a risk calculator that indicates risk in the form of a thermometer rather than paper charts, highlighting how a visual thermometer is more appealing, easier to understand and can even be motivational, although anxiety may be evoked for those whose risk is very high [54]. A qualitative study conducted in the UK to understand the experiences of participants presented with a personalised risk report that includes heart age and QRISK2 risk score that predicts a person’s risk of having a stroke or heart attack within the next 10 years, finds that patients tend to recall heart age easier rather than a probabilistic score [52].
Regarding format preferences, a cross-sectional study examining the preferences of patients attending GP practices in Auckland, New Zealand found that relative risk (n = 603/934, 64.5%) was the highest ranked mode of risk presentation preferred, followed by absolute risk (n = 131/934, 14.0%), then natural frequencies (n = 91/934, 9.7%), when it comes to the format that would help a patient to decide. In this study, the relative risk was ranked first by participants who were more numerate (OR = 1.2; 95% CI, 1.0–1.4), by those who were more concerned about a heart attack (OR = 1.1; 95% CI, 1.01–1.2), and less by Pacific Islanders (OR = 0.4) or Asian (OR = 0.4) participants (ethnicity overall, 95% CI, 0.7–0.8). Pictures were preferred over numbers by those who had less schooling (OR = 1.2; CI, 1.1–1.3) and by those who were less numerate (OR = 1.1; CI, 1.01–1.2) [51].
In a multi-component RCT conducted in Egypt to investigate the accuracy of CVD risk perception among patients with diabetes, patients were provided a combination of absolute and relative risk scores conveyed in percentage and frequency formats, and given advice framed positively by physicians on how to change their risk based on WHO/ISH guidelines. Agreement between perceived and objective CVD scores increased substantially for the intervention group (n = 107) (pre-/post- intervention, kappa = 0.271 ± 5.2%, p = 0.0 to 0.837 ± 4.4%, p = 0.0), compared to the control group (kappa = 0.088/ ± 4.5%, p = 0.052 to 0.105 ± 4.6%, p = 0.022), which increased only marginally and remained low [60].
Perception of risk information over time
Two intervention studies suggest that a patient’s perception of risk, although mediated by formatting and visualisation elements that aim to improve understanding, tends to taper off after a period of time. A non-inferiority RCT conducted in Germany to test a time-to-event (TTE) format versus emoticons in representing a patient’s 10-year absolute risk of CVD finds TTE to have a stronger effect on risk perception than emoticons [41], although the effect on perception waned somewhat after 3 months [42]. Another RCT intervention conducted with type 2 diabetes patients newly referred to a diabetes care system in the Netherlands, that used a 6-step CVD risk communication method, found that patients in the intervention group were able to estimate their risk of developing CVD more accurately than those in the control group in the short term (appropriateness of risk perception, intervention 0.33 vs. control − 0.1, difference = 0.48, CI 0.02 to 0.95 (p = 0.04)), but the effect of risk perception diminished after 12 weeks. The intervention used a combination of tools that included conveying to patients their absolute risk scores calculated using the UK prospective diabetes study (UKPDS) risk engine, together with a risk card with a population diagram, and having positively framed risk messages conveyed to patients [62].
Diabetes-related risk information
There were only a small number of studies on patient-physician risk communication focused on diabetes. Five articles, consisting of 2 qualitative [66, 70] and 3 quantitative studies [65, 68, 69], focused on how sufficient information about diabetes-related complications and risks were not conveyed adequately by physicians.
Understanding and recalling risk information
One descriptive cross-sectional study conducted at a diabetes centre describe how only about 23% (32/138) and 14% (19/138) of patients diagnosed with diabetes respectively recall their providers providing them with factual information and warning them about the implications of complications [68]. The low proportion seem to imply a relatively limited number of patients who are conveyed actual risk of complications by their physicians. A focus group discussion conducted with type 2 diabetes patients from community health service centres in Guangzhou, China, describes how patients understand normal blood glucose and HbA1c levels as reflective of a stable condition, and that higher numbers or fluctuating numbers are a source of worry. Though they were concerned about diabetes complications, patient participants were not aware that diabetes was a risk factor for CVDs. Patients found consultations with physicians to be too brief and wanted more information about how diabetes can progress and develop further into complications [70]. Physicians who treat patients with diabetes, describe engaging more with communicatively active patients (CAP) who are able to recognise and respond to new or evolving medication risk information [66].
One pilot RCT study with diabetes patients assessing the feasibility of adopting a new risk communication intervention tool, based on behavioural and psychological concepts in primary care and focusing on diabetes as a risk factor for CVD, found that recall for effective heart age is significantly better than other formats such as 10-year CVD risk, both immediately and 12 weeks after intervention [69]. Another pilot intervention conducted at 2 clinics in the University of Chicago, USA, found that using a web-based decision support tool can improve risk understanding. The intervention consists of an education support module, a model for calculating life expectancy and risk of developing CVDs, a treatment preference questionnaire, and a geriatric screening component consolidated in the form of a personalised report to be delivered to patients prior to a patient’s visit with a physician. The study showed that decisional conflict (DC) is reduced in the intervention group more than in the control group (overall DC score pre-/post-intervention 52.7 ± 33.0 to 24.5 ± 26.7, pre-/post-control 51.2 ± 35.5 to 36.6 ± 33.8, p = 0.07). Although the results for the overall DC scale were not significant, the informed DC subscale which asks about knowledge and risk understanding related to A1c goals was significant (Informed DC subscale score pre-/post-intervention 54.0 ± 40.1 to 18.3 ± 33.7, pre-/post-control 56.0 ± 38.4 to 31.0 ± 41.0, p ≤ 0.001) [65].
Strategies and approaches used by physicians in patient-physician risk communication
Eight articles (5 CVD, 3 diabetes), consisting of 7 qualitative [43, 45, 46, 53, 58, 66, 70] and 1 quantitative study [68], describe strategies and approaches used by physicians to communicate risk information to patients. Strategies used to convey CVD risk include the use of fear or scare tactics [45, 46, 53], the use of strong language to evoke fear [58], the use of positive language [45], downplaying risk [53], or the use of metaphors and analogies to simplify risk information and improve patient's understanding, such as associating heart function with an electrical system [46]. Other approaches used include presenting different CVD scenarios to those who are less adherent to medication [43], speaking indirectly to patients [45], intervening strategically during ‘teaching’ moments [58], emphasising gradual and continuous change [58], and prioritising discussion points [58].
For patients with diabetes, strategies used by physicians include setting goals [70], using specific words such as ‘common’ or ‘rare’ to describe risks [66], avoiding statistics [66], varying presentation styles to different types of patients [66], and withholding information with low-level risks that may affect a patient’s medication intake [66]. Additional approaches include using dramatic images such as illustrations of amputations to persuade patients [70]. Physicians who provided prompt feedback to patients using clear language and positive body language were viewed positively by patients [70]. Diabetes patients, like patients treated for CVD conditions, similarly mentioned how physicians used fear as a motivator to warn about complications [68].
The use of fear and scare tactics was a recurring theme used by physicians to direct patients towards desired behaviour. If physicians perceived patients to be of higher risk but generally unmotivated about their own health [45], the consequences of risky behaviour and complications that can occur in the future (e.g., such as being bed bound) were used to persuade patients to change [46, 58, 68]. If patients were not ready and receptive to risk information, then physicians would avoid conveying risks directly to avoid alarming or affecting patients negatively [45]. On the contrary, for patients who were motivated or self-activated, physicians use a positive strategy to reassure and encourage patients to focus on achievable change [45], since a communicatively active patient is cognitively ready to receive more information about risks [66].
A complete summary of strategies and approaches used by physicians for CVDs and diabetes-related conditions is described in Table 4.
Communication tools that facilitate patient-physician risk communication
Eight articles (7 CVD, 1 diabetes), consisting of 6 qualitative [43,44,45,46, 52, 61], 1 quantitative [67] and 1 mixed methods study [54], identify tools used to facilitate the communication of CVD risks between patients and physicians. Studies describe the use of personalised risk reports to aid the understanding of risks [52], that contain pictorial information such as colour codes to indicate CVD risk status with visual depictions of arterial plaques [44]. Other tools used include risk charts [45, 54], risk tables [61], drawn diagrams [46], CVD guidelines [43], risk assessment tools [43], results from risk calculators such as the JBS2 calculator in the UK [45, 54], and images such as cholesterol spikes or how the brain looks like during a stroke [45]. For communication tools used by diabetes patients, 1 study found that although most risk factor discussion with physicians still occurs in-person, those who use both phone and messages to communicate risk factors tend to also have higher in-person provider visits, insulin use and poorly controlled Hba1c [67].
Interventions to improve the communication of risk information
Interventions related to CVD conditions
Eight interventions were related to CVDs, of which 6 were RCTs [41, 42, 55, 57, 60, 62], 1 a controlled trial without randomisation [48], and 1 an interrupted time series design [63]. Most interventions used a combination of training and interventional materials for physicians or healthcare providers, along with reinforcing tools provided to patients that emphasise risk management. Interventions usually involve the use of relative and absolute CVD risk scores generated by risk calculators or algorithms [55, 60, 62, 63], or reports that present CVD risk stratification using colour codes [48]. A pocket guideline from the National Health Cholesterol Education Program and WHO/ISH guidelines were used by Casebeer et al. (2009) [48] and Tawfik et al. (2016) [60] respectively. Additional intervention tools include the use of scripts like decision aid [55], use of smiley faces [55], emoticons or time-to-event graphics to visualise risk [41, 42], population diagrams [62], positive framing [62], risk cards [62], educational worksheets [63], checklists [63], patient contract or pledges [48], and chart stickers [48]. 1 study used a tablet with multiple educational modules, linked to a patient’s electronic health records (EHR), that requires a patient to access and watch before attending a consultation session with a physician, spread over a period of 5 visits [57].
Five interventions [48, 55, 57, 62, 63] required patients to actively respond to the risk information and educational materials provided to them by a physician or health professional. This includes pledging to commit to the medical regimen (statin therapy) of the intervention [48], participating in a shared decision-making process with the physician in modifying one’s risk [55], talking to the physician shortly after watching educational modules on a tablet [57], thinking aloud to check if one can explain one’s own risk [62], or completing a checklist to count the number of tick marks one has while assessing the risk category one falls into [63]. 1 intervention study, the Heart Health Counts program, sent 5 print mailings over a 4-month period to patients who are new to statin therapy with information focusing on various aspects of CVD risk [48].
Before the start of each intervention, training or meeting sessions are sometimes initiated between physicians and other healthcare professionals to familiarise on study protocol and aims [63], discuss the epidemiology of CVD risk calculations [55], elaborate on the meaning of absolute and relative risks [60] and discuss practical strategies of how to communicate risk information to patients [55, 60, 62, 63]. Sessions are also held to train physicians and healthcare professionals on how to use risk prediction tools such as the adapted Framingham algorithm [55], WHO/ISH CV risk prediction chart [60], or the UKPDS risk engine [62]; to discuss the causes and consequences of CVD risk [62] and discuss the ethics of shared decision making [55]. For 1 intervention, training included explaining behavioural theories such as the Theory of Planned Behaviour and Self-Regulation Theory to physicians [60], although engagement with theoretical concepts was not common among most interventions included in this review. Role-playing was included in some of the intervention training sessions to reinforce learning [55, 63]. Other than a general outline of training procedures, the interventions described do not provide more details regarding the process and flow of training sessions.
Interventions related to diabetes
3 interventions were related to diabetes, consisting of 3 RCTs [64, 65, 69]. All the interventions used a personalised decision aid or support tool to present risks to patients in the form of a report [64, 65, 69]. 1 pilot intervention was specifically guided by ideas from behavioural economics and psychology to shape the design of the intervention, factoring in concepts such as optimistic bias, affect or representative heuristic, risk aversion, present biases and limited attention span to determine the structure of risk format and description of risk information [69]. The intervention study, however, did not go into detail about how concepts are translated into risk information. Personalised reports usually include a patient’s screening test results [64, 65], treatment options or recommended actions that should be taken for specific risk factors [64, 69]. Reports also usually include a calculator that estimates life expectancy or risk of developing complications such as developing a heart attack or risk of amputation or blindness [65], or that estimates heart age [69], with an education module [65]. Risks are described using natural frequencies to convey outcome probabilities relevant to each patient [64, 65, 69]. 1 intervention mentioned setting achievable goals for patients to aim towards to [64], while 2 interventions required patients to discuss their treatment options with a physician after a personalised report was received by the patient [64, 65].
A summary of intervention components and associated studies is described in Table 5.
Health literacy and communication effectiveness assessment in interventions
Interventions related to CVD conditions
Of eight interventions related to CVDs, only 3 studies measured health literacy [55, 57, 63], while 5 studies did not use any health literacy or related measurement tools during the course of interventions [41, 42, 48, 60, 62]. Krones et al. (2008) [55] asked 3 questions on CVD prevention that was measured post-intervention with patients but did not specify what each specific question was. Similarly, Roach et al. (2010) [57] measured “patient knowledge and perception regarding the presence of CVD risk and risk factors” during patients’ first consultation visit, but did not specify what scales or questions were used. Williams et al. (2016) [63] asked 6 questions related to stroke knowledge and associated risk factors, which together formed a ‘knowledge’ index, that was administered to patients after intervention.
To determine whether interventions made a difference in patient-physician communication, or communication with a healthcare professional, 4 out of 8 intervention studies related to CVDs report using measurements to assess whether communication improved [57, 60, 62, 63]. Three studies did not use any measurements at all [41, 42, 48], while 1 study used a scale (the patient participant scale) that the study team was unable to locate at the source article and thus determine if communication effectiveness was measured [55].
Roach et al. (2010) [57] used descriptive questions to check if interventions increased discussion about chronic diseases during consultations. Questions include whether heart attack risk, lowering cholesterol and smoking cessation were specifically discussed during each intervention visit. Tawfik et al. (2016) [60] measured cardiovascular risk perception and accuracy at baseline and at 3 months after intervention. Participants were asked to self-rate their own risk of developing heart disease within 10 years, which was subsequently compared to their actual risk. Welschen et al. (2012) [62] also asked participants to self-rate their risk of developing CVD in 10 years, at 2 to 12 weeks after intervention. Additionally, the Combined outcome measure for risk communication and treatment decision-making effectiveness (COMRADE) ‘satisfaction with communication’ sub-scale was used but mainly regarding interaction with diabetes nurses. Williams et al. (2016) [63] used a ‘discussion’ and ‘recommendation’ index to measure if stroke-related risk factors were discussed with physicians, and whether any recommendations were made.
Interventions related to diabetes
Of three studies related to Diabetes, none reported assessing for health literacy [64, 65, 69]. 2 studies used some form of measurement to check for the effectiveness of communication [65, 69], while 1 study did not assess communication effectiveness at all [64]. In the pilot RCT conducted by Huang et al. (2016) [65], although not specifically about risks, communication about A1C goals was included pre- and post-intervention, as part of the overall decisional conflict scale. Rouyard et al. (2018) [69] mentioned using an adapted COMRADE scale to assess ‘participant’s satisfaction’ as a primary outcome but did not mention which parts of the scale were adapted.
Barriers and facilitators to interventions
Interventions related to CVD conditions
Of eight interventions related to CVDs, 1 study highlighted barriers to interventions [63], 4 studies mentioned facilitators [48, 55, 60, 62], while 3 studies did not mention barriers or facilitators [41, 42, 57]. Barriers mentioned include physicians being overly burdened by the requirements of the intervention, thus not being able to apply the interventions consistently. In discussing stroke risk with patients using a checklist, physicians started limiting conversations to about 10 patients a day, then subsequently communicated less with patients as the study progressed due to a lack of staff and time for each individual patient, as well as poor motivation and lack of buy-in from physicians and nurses [63]. Other barriers include overcrowded and inadequate facilities [63].
Facilitators to interventions include using a multifaceted implementation strategy involving education seminars, printed materials and consultation aids, and incorporating local leaders as part of a multi-step intervention to deliver subjective and objective risk to patients [55]. Other facilitators mentioned include not having the implementation being labour intensive [48], repeating CVD risk communication with patients [60], targeting interventions to those who are newly diagnosed with diabetes [62], and focusing on patients who are already committed to preserving their own health [48]. Welschen et al. (2012) [62] mentioned how engagement with behavioural theories improved the overall effectiveness of intervention by aligning research questions and study methods with outcome measures.
Interventions related to diabetes
Of three intervention studies related to diabetes, 2 studies highlighted barriers related to the implementation of the intervention [64, 69], and 1 study did not mention any barriers or facilitators [65]. Barriers include healthcare providers not being accustomed to the decision aid used as an intervention, using the decision aid only once, perceiving the intervention as unneeded, and not using the intervention as intended for a substantial number of patient participants [64]. Other study-related barriers include implementing the intervention at a single site and having only a short time to follow-up with participants to determine if the intervention has any substantive effects on health outcomes [69].
Discussion
Physicians employ a wide variety of strategies and tools to communicate risk depending on a patient’s attributes and responses. Strategies used by physicians aim to uncover a patient’s ability to understand risk information and at the same time, convince or persuade patients who are less self-activated to gravitate more positively towards behaviour modification. Tools such as calculator-generated risk scores aim to provide generalised estimates for patients to better understand their own risk profile, while risk reports and other associated toolkits help simplify information, consolidate personal health details, and ease the process of communication so that knowledge can be transmitted more accessibly. In the context of CVDs and diabetes, this involves motivating patients while delicately conveying the probability of negative consequences in the medium to long term.
For CVDs, some communication approaches used by physicians such as downplaying risk or using analogies to describe risk, suggest avoiding or delaying the conveyance of actual risk is a strategy used by physicians, particularly for less receptive patients. An implication is whether such a strategy will lead to an underestimation of risk or risk avoidance by both physicians and patients during consultation sessions. Other methods such as communicating indirectly and emphasising gradual change aim to redirect the focus of patients while still communicating risk, while presenting different CVD scenarios involves offering counterfactual scenarios for patients to consider why specific actions should be taken and what can be done to reduce risk factors. An ongoing challenge is whether appealing to such comparative scenarios can convince patients to modify their behaviour.
For diabetes risk communication approaches, studies by Ledford (2011) [66] and Yao et al. (2022) [70] point to potential discordance between what patients want and what physicians are willing to convey. Ledford (2011) [66] discusses how physicians try to avoid statistics and use simple words such as ‘common’ and ‘rare’ to simplify risk information for the communicatively ‘non-active’ patient. In Yao et al. (2022) [70] on the other hand, patients mentioned how encounters with physicians were too short and how they were reprimanded for asking too many questions. Patients likewise felt that they were provided insufficient explanations about their prescriptions [70]. The 2 articles highlight potential trade-offs that physicians need to make while simplifying risk information, where details may need to be parsed at the expense of comprehensiveness. A pertinent question is how information can be abridged without compromising depth while retaining key messages in addition to the conveyance of risk.
One discernible risk communication strategy related to both CVDs and diabetes is the use of fear to persuade or motivate patients. Rather than evoking an emotive state in patients, fear is used strategically at different junctures by physicians to try and change a patient’s behaviour. Physicians use fear to raise a sense of urgency in high-risk, unmotivated patients; to warn patients of inaction, to encourage patients to imagine a worser physiological state in the future to ‘jolt’ them out of their current circumstances, or simply to force patients to think about closely associated consequences, such as physiological incapacitation. However, it remains to be seen if fear used as a communication approach can be sustainable over the medium to long term. For chronic disease management, patients need to be persistent and conscientious in managing their own health. Using scare tactics may inure patients once they become accustomed to the same type of fear messages repeated multiple times.
Given the diverse approaches among current practices and the range of tools available, there is a need to combine implementation with behavioural or psychological concepts within risk communication practices that can be sustained over the longer term [76,77,78,79]. The range of studies included in this review generally did not report or engage extensively with implementation science principles or behavioural science concepts. Only 1 study was guided by conceptual ideas from behavioural psychology [69], and 2 intervention studies incorporated psychological theories as part of training for physicians before the implementation of the intervention [60, 62]. Engaging with implementation science and behavioural concepts can ensure that strategies are carefully integrated so that approaches and interventions can gain traction with multi-stakeholder groups and can be viable over the longer term [80,81,82]. Interventions can be resource and time-consuming, requiring the training of personnel, the use of expertise, and inter-professional collaboration, all of which can affect the existing configuration of healthcare processes. A thoughtful approach would identify prevailing gaps, iteratively test for different design features, and incorporate feedback mechanisms in tracking the effectiveness of different risk communication approaches to aim for longer-term impact.
Recent trends have emphasised a shift in patient-physician consultations away from a paternalistic model towards a shared decision-making or patient-centred model of clinical care [83], where a patient is viewed as an equal partner in patient-physician interaction actively involved in one’s own management of health [84, 85]. In shared decision-making, patients participate in a deliberative process with their primary physician in making informed choices based on a patient’s values and preferences [86]. The articles included in this systematic scoping review point to some of the salient challenges in shifting towards such a model and focus on some of the contextual difficulties and implementation challenges in communicating risk information.
Gaps in the literature
Articles included in this review underscore some of the fundamental gaps in the existing literature. One notable gap is a lack of studies describing the development of risk information tools in stepwise detail at the pre-intervention stage. Particularly, studies presenting the rationale of why preferred risk information is chosen in the first place, and how information is piloted with users before implementation or actual use. Understanding risk tools and how it is connected from conception to implementation, can help in the translation and replication of comparable tools across populations, and aid physicians in communicating risk effectively. One example related to the translation of tools for use is the visual positioning of graphs, symbols, numbers, frequencies or text in different parts of a report. Positioning, in synchronisation with visual or graphical formats and textual information, can influence the uptake and acceptability of risk reports, especially for physicians with limited time, who need to communicate to a wide range of patients daily.
Studies included in this review generally did not measure cultural or linguistic components quantitatively or qualitatively, which is a major shortcoming. It is well documented that for both CVDs and diabetes, specific ethnic groups are at higher risk of developing complications due to a combination of socio-economic and contextual factors [87,88,89,90]. For instance, for CVDs in the United States, ethnic minorities diagnosed with hypertension are less likely to achieve control and more likely to experience end-stage organ failure, while African American patients diagnosed with dyslipidemia are less likely to be prescribed lipid-lowering therapy than patients who are white [91]. For diabetes, African Americans and Hispanics with diabetic retinopathy have a higher likelihood of progressing to blindness or visual impairment than white populations [92].
With the exception of Hawking et al. (2019) [52] in the UK, whose study participants are mainly from Black and South Asian minority groups, and Yao et al. (2022) [70], whose study was conducted in China and generated cultural related themes, such as the use of traditional Chinese medicine among patients and language barriers due to dialect speaking patients, most qualitative studies included in this review did not consider cultural or linguistic factors as a significant consideration in the risk communication process. Ethnic disparities in the progression of chronic diseases suggest greater understanding is needed on how to tailor risk information to different ethnic groups and communities, that accounts for cultural practices, linguistic conventions, and structural context that can increase patient engagement at the individual and community level [87, 88, 93].
Another gap is the examination of risk communication as it occurs in situ within the clinical setting. If risk communication processes are to be understood naturalistically in daily practice, then prospective data collection methods that rely only on patient’s or physician’s recall may not fully capture the diverse range of approaches that physicians and patients respond to risk information. It is likely that for pragmatic reasons, the traditionally closed set-up of the clinic makes it harder for researchers to observe ongoing clinical encounters, but capturing processes as closely to the clinical setting as possible can allow one to examine processes of communication more intricately.
Some recent studies have used video, audio recordings, or participant observations to capture clinical conversations in real time for useful analysis. A comprehensive study by Gidlow et al. (2021) [94] for instance, conducted in 12 general practices in the West Midland of England, examined how healthcare assistants or practice nurses communicate cardiovascular risk to patients using the QRISK2 or JBS3 CVD calculator through video recordings, which were subsequently systematically coded and used for simulated recall interviews with patients and practitioners. A diabetes study by Kruse et al. (2013) [95], although not specifically about the conveyance of risk, that used audio-recorded clinical encounters for analysis, uncovered how patients find it difficult to relate to physicians’ emphasis on quantitative health outcomes during clinical conversations given caregiving and work commitments that patients have.
As shown by the overall quantity of articles related to CVDs as compared to diabetes, there is an overall lack of studies examining the communication of risk for patients with diabetes. This may be explained by the more conventional use of risk calculators within the clinical setting for CVDs in helping higher risk patients understand their own risk levels that are based on well-established longitudinal studies, and due to the complexity of diabetes that is caused by a multitude of lifestyle-related risk factors such as diet and levels of exercise which are variable and harder to calculate as an aggregate risk. Two pilot-controlled trials for diabetes included in this review have small sample sizes that may require further larger studies to validate the effectiveness of the interventions used.
Strengths and limitations
The strength of this review is to provide a comprehensive and detailed overview of studies that examine patient-physician risk communication in the primary or secondary care setting, for both CVDs and diabetes. Although the range of studies is heterogeneous in focusing on different aspects of risk communication and interventions, the studies collectively highlight some of the common issues that arise and gaps in the literature. Qualitative studies provide depth in uncovering the underlying complexities articulated by patients and physicians while communicating risk information with one another. Quantitative studies show some of the risk format preferences or multi-faceted interventions that have been attempted to improve the communication of risks in the clinical setting, as well as the effectiveness of interventions.
This review focuses on the encounter between physicians and patients as it is the most conventional means by which risk information is communicated. However, in doing so, we have not included studies in which patients are given health risk information by other healthcare professionals, such as nurse practitioners, pharmacists, dieticians, psychologists, and community health workers, who often may also be closely involved in the overall management of a patient’s care where risk information is routinely conveyed.
Another limitation of this review is the exclusion of studies that examine risk communication in populations that do not involve a patient-physician encounter. Given the limited amount of time and interspersed nature of the physician encounter for most non-acute patients, a patient’s notion of risk may invariably be influenced by what occurs prior to or after an encounter with a physician. A patient’s background, disposition, attitudinal factors, and health literacy, interacting with outside sources of risk information, can augment or attenuate a patient’s acceptance and understanding of risk.
Lastly, a further limitation of this review is the exclusion of review studies, studies that were conducted before 2000, and the exclusion of foreign language studies during the screening process, which limits the range of articles that were screened for this review.
Conclusion
While multiple research studies have been conducted that explicate various ways in which physicians communicate risk to patients, there is a critical need for further research on risk communication strategies related to CVDs and diabetes. Research should focus on developing targeted approaches that enhance patients' understanding of their risk profiles. Recommendations should provide evidence-based guidelines to assist physicians and healthcare professionals improve risk communication within clinical settings, with the goal of facilitating patient comprehension that can support sustained lifestyle and behavioural changes through informed, evidence-based methods. Misalignment in communication may lead to confusion, dissatisfaction, and lack of clarity, which may impede the effective management of chronic conditions.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AAFP:
-
American Association of Family Physicians
- AF:
-
Atrial fibrillation
- COMRADE:
-
Combined outcome measure for risk communication and treatment decision making effectiveness
- CVD:
-
Cardiovascular disease
- DC:
-
Decisional conflict
- GPs:
-
General practitioners
- JBS 3:
-
Joint British Societies risk calculator
- MeSH:
-
Medical subject headings
- MMAT:
-
Mixed method appraisal tool
- NHS:
-
National Health Service
- RCT:
-
Randomised controlled trial
- TIA:
-
Transient ischaemic attack
- TTE:
-
Time to event
- UKPDS:
-
United Kingdom prospective diabetes study
- WHO/ISH:
-
World Health Organisation/International Society of Hypertension
References
DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. https://doiorg.publicaciones.saludcastillayleon.es/10.1038/nrdp.2015.19.
Dokken BB. The Pathophysiology of Cardiovascular Disease and Diabetes: Beyond Blood Pressure and Lipids. Diabetes Spectrum. 2008;21(3):160–5. https://doiorg.publicaciones.saludcastillayleon.es/10.2337/diaspect.21.3.160.
American Diabetes Association Professional Practice C. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S20-S42. https://doiorg.publicaciones.saludcastillayleon.es/10.2337/dc24-S002
Marx N, Federici M, Schutt K, Muller-Wieland D, Ajjan RA, Antunes MJ, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44(39):4043–140. https://doiorg.publicaciones.saludcastillayleon.es/10.1093/eurheartj/ehad192.
Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023;148(9):e9–119. https://doiorg.publicaciones.saludcastillayleon.es/10.1161/CIR.0000000000001168.
American Diabetes Association Professional Practice C. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S111-S25.
Zhang X, Li L, Zhang Q, Le LH, Wu Y. Physician Empathy in Doctor-Patient Communication: A Systematic Review. Health Commun. 2024;39(5):1027–37. https://doiorg.publicaciones.saludcastillayleon.es/10.1080/10410236.2023.2201735.
Hinder S, Greenhalgh T. "This does my head in". Ethnographic study of self-management by people with diabetes. BMC Health Services Research. 2012;12(1):83. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/1472-6963-12-83
Bonds DE, Camacho F, Bell RA, Duren-Winfield VT, Anderson RT, Goff DC. The association of patient trust and self-care among patients with diabetes mellitus. BMC Fam Pract. 2004;5:26. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/1471-2296-5-26.
Becker ER, Roblin DW. Translating primary care practice climate into patient activation: the role of patient trust in physician. Med Care. 2008;46(8):795–805. https://doiorg.publicaciones.saludcastillayleon.es/10.1097/MLR.0b013e31817919c0.
Wollny A, Altiner A, Daubmann A, Drewelow E, Helbig C, Loscher S, et al. Patient-centered communication and shared decision making to reduce HbA1c levels of patients with poorly controlled type 2 diabetes mellitus - results of the cluster-randomized controlled DEBATE trial. BMC Fam Pract. 2019;20(1):87. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12875-019-0977-9.
Beck RS, Daughtridge R, Sloane PD. Physician-patient communication in the primary care office: a systematic review. J Am Board Fam Pract. 2002;15(1):25–38.
Larsen KM, Smith CK. Assessment of nonverbal communication in the patient-physician interview. J Fam Pract. 1981;12(3):481–8.
Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139(11):907–15. https://doiorg.publicaciones.saludcastillayleon.es/10.7326/0003-4819-139-11-200312020-00009.
Shen MJ, Peterson EB, Costas-Muniz R, Hernandez MH, Jewell ST, Matsoukas K, et al. The Effects of Race and Racial Concordance on Patient-Physician Communication: A Systematic Review of the Literature. J Racial Ethn Health Disparities. 2018;5(1):117–40. https://doiorg.publicaciones.saludcastillayleon.es/10.1007/s40615-017-0350-4.
Williams MV, Davis T, Parker RM, Weiss BD. The role of health literacy in patient-physician communication. Fam Med. 2002;34(5):383–9.
Sudore RL, Landefeld CS, Perez-Stable EJ, Bibbins-Domingo K, Williams BA, Schillinger D. Unraveling the relationship between literacy, language proficiency, and patient-physician communication. Patient Educ Couns. 2009;75(3):398–402. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.pec.2009.02.019.
Cavanaugh K, Huizinga MM, Wallston KA, Gebretsadik T, Shintani A, Davis D, et al. Association of numeracy and diabetes control. Ann Intern Med. 2008;148(10):737–46. https://doiorg.publicaciones.saludcastillayleon.es/10.7326/0003-4819-148-10-200805200-00006.
Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health. 2004;94(12):2084–90. https://doiorg.publicaciones.saludcastillayleon.es/10.2105/ajph.94.12.2084.
Alexander GC, Casalino LP, Meltzer DO. Patient-Physician Communication About Out-of-Pocket Costs. JAMA. 2003;290(7):953–8. https://doiorg.publicaciones.saludcastillayleon.es/10.1001/jama.290.7.953.
Larson EB, Yao X. Clinical empathy as emotional labor in the patient-physician relationship. JAMA. 2005;293(9):1100–6. https://doiorg.publicaciones.saludcastillayleon.es/10.1001/jama.293.9.1100.
Dugdale DC, Epstein R, Pantilat SZ. Time and the patient-physician relationship. J Gen Intern Med. 1999;14 Suppl 1(Suppl 1):S34–40. https://doiorg.publicaciones.saludcastillayleon.es/10.1046/j.1525-1497.1999.00263.x
Ratanawongsa N, Roter D, Beach MC, Laird SL, Larson SM, Carson KA, et al. Physician burnout and patient-physician communication during primary care encounters. J Gen Intern Med. 2008;23(10):1581–8. https://doiorg.publicaciones.saludcastillayleon.es/10.1007/s11606-008-0702-1.
Yuguero O, Marsal JR, Esquerda M, Soler-Gonzalez J. Occupational burnout and empathy influence blood pressure control in primary care physicians. BMC Fam Pract. 2017;18(1):63. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12875-017-0634-0.
Ha JF, Longnecker N. Doctor-patient communication: a review. Ochsner J. 2010;10(1):38–43.
Schulberg SD, Ferry AV, Jin K, Marshall L, Neubeck L, Strachan FE, et al. Cardiovascular risk communication strategies in primary prevention. A systematic review with narrative synthesis. J Adv Nurs. 2022;78(10):3116–40. https://doiorg.publicaciones.saludcastillayleon.es/10.1111/jan.15327
Mentrup S, Harris E, Gomersall T, Kopke S, Astin F. Patients’ Experiences of Cardiovascular Health Education and Risk Communication: A Qualitative Synthesis. Qual Health Res. 2020;30(1):88–104. https://doiorg.publicaciones.saludcastillayleon.es/10.1177/1049732319887949.
Waldron CA, van der Weijden T, Ludt S, Gallacher J, Elwyn G. What are effective strategies to communicate cardiovascular risk information to patients? A systematic review Patient Educ Couns. 2011;82(2):169–81. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.pec.2010.04.014.
Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013;2013: 653789. https://doiorg.publicaciones.saludcastillayleon.es/10.1155/2013/653789.
Dal Canto E, Ceriello A, Ryden L, Ferrini M, Hansen TB, Schnell O, et al. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26(2_suppl):25–32. https://doiorg.publicaciones.saludcastillayleon.es/10.1177/2047487319878371
Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20(10):685–95. https://doiorg.publicaciones.saludcastillayleon.es/10.1038/s41569-023-00877-z.
Asimakopoulou K. Risk communication in type 2 diabetes. J Diab Nursi. 2008;12(5):178–86. Available from: https://diabetesonthenet.com/wp-content/uploads/jdn12-5pg178180-181184-6-1.pdf
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. https://doiorg.publicaciones.saludcastillayleon.es/10.1080/1364557032000119616.
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/1472-6947-7-16.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doiorg.publicaciones.saludcastillayleon.es/10.1136/bmj.n71.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73. https://doiorg.publicaciones.saludcastillayleon.es/10.7326/M18-0850.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s13643-016-0384-4.
American Academy of Family Physicians. Primary Care. Aafp.org. American Academy of Family Physicians; 2017. Available from: https://www.aafp.org/about/policies/all/primary-care.html
World Health Organization. Number of outpatient care facilities. WHO Global Health Observatory. Available from: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/1348
World Health Organization. Number of outpatient visits per person per year. WHO Global Health Observatory. Available from: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3117
Adarkwah CC, Jegan N, Heinzel-Gutenbrunner M, Kuhne F, Siebert U, Popert U, et al. Time-to-event versus ten-year-absolute-risk in cardiovascular risk prevention - does it make a difference? Results from the Optimizing-Risk-Communication (OptRisk) randomized-controlled trial. BMC Med Inform Decis Mak. 2016;16(1):152. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12911-016-0393-1.
Adarkwah CC, Jegan N, Heinzel-Gutenbrunner M, Kuhne F, Siebert U, Popert U, et al. The Optimizing-Risk-Communication (OptRisk) randomized trial - impact of decision-aid-based consultation on adherence and perception of cardiovascular risk. Patient Prefer Adherence. 2019;13:441–52. https://doiorg.publicaciones.saludcastillayleon.es/10.2147/PPA.S197545.
Barfoed BL, Jarbol DE, Paulsen MS, Christensen PM, Halvorsen PA, Nielsen JB, et al. GPs’ Perceptions of Cardiovascular Risk and Views on Patient Compliance: A Qualitative Interview Study. Int J Family Med. 2015;2015: 214146. https://doiorg.publicaciones.saludcastillayleon.es/10.1155/2015/214146.
Bengtsson A, Lindvall K, Norberg M, Fharm E. Increased knowledge makes a difference! - general practitioners’ experiences of pictorial information about subclinical atherosclerosis for primary prevention: an interview study from the VIPVIZA trial. Scand J Prim Health Care. 2021;39(1):77–84. https://doiorg.publicaciones.saludcastillayleon.es/10.1080/02813432.2021.1882083.
Bonner C, Jansen J, McKinn S, Irwig L, Doust J, Glasziou P, et al. Communicating cardiovascular disease risk: an interview study of General Practitioners’ use of absolute risk within tailored communication strategies. BMC Fam Pract. 2014;15:106. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/1471-2296-15-106.
Borg Xuereb C, Shaw RL, Lane DA. Patients’ and physicians’ experiences of atrial fibrillation consultations and anticoagulation decision-making: A multi-perspective IPA design. Psychol Health. 2016;31(4):436–55. https://doiorg.publicaciones.saludcastillayleon.es/10.1080/08870446.2015.1116534.
Bridgwood BM, Wilson A, Clarke D, Eborall H. Understanding TIA: an ethnographic study of TIA consultations. Fam Pract. 2020;37(4):530–4. https://doiorg.publicaciones.saludcastillayleon.es/10.1093/fampra/cmaa004.
Casebeer L, Huber C, Bennett N, Shillman R, Abdolrasulnia M, Salinas GD, et al. Improving the physician-patient cardiovascular risk dialogue to improve statin adherence. BMC Fam Pract. 2009;10:48. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/1471-2296-10-48.
Durack-Bown I, Giral P, d’Ivernois JF, Bazin C, Chadarevian R, Benkritly A, et al. Patients’ and physicians’ perceptions and experience of hypercholesterolaemia: a qualitative study. Br J Gen Pract. 2003;53(496):851–7.
Fisseni G, Lewis DK, Abholz HH. Understanding the concept of medical risk reduction: a comparison between the UK and Germany. Eur J Gen Pract. 2008;14(3–4):109–16. https://doiorg.publicaciones.saludcastillayleon.es/10.1080/13814780802580247.
Goodyear-Smith F, Kenealy T, Wells S, Arroll B, Horsburgh M. Patients’ preferences for ways to communicate benefits of cardiovascular medication. Ann Fam Med. 2011;9(2):121–7. https://doiorg.publicaciones.saludcastillayleon.es/10.1370/afm.1193.
Hawking MKD, Timmis A, Wilkins F, Potter JL, Robson J. Improving cardiovascular disease risk communication in NHS Health Checks: a qualitative study. BMJ Open. 2019;9(8): e026058. https://doiorg.publicaciones.saludcastillayleon.es/10.1136/bmjopen-2018-026058.
Honey S, Hill K, Murray J, Craigs C, House A. Patients’ responses to the communication of vascular risk in primary care: a qualitative study. Prim Health Care Res Dev. 2015;16(1):61–70. https://doiorg.publicaciones.saludcastillayleon.es/10.1017/S1463423613000509.
Kirby M, Machen I. Impact on clinical practice of the Joint British Societies’ cardiovascular risk assessment tools. Int J Clin Pract. 2009;63(12):1683–92. https://doiorg.publicaciones.saludcastillayleon.es/10.1111/j.1742-1241.2009.02201.x.
Krones T, Keller H, Sonnichsen A, Sadowski EM, Baum E, Wegscheider K, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Ann Fam Med. 2008;6(3):218–27. https://doiorg.publicaciones.saludcastillayleon.es/10.1370/afm.854.
Polak L, Green J. Using quantitative risk information in decisions about statins: a qualitative study in a community setting. Br J Gen Pract. 2015;65(633):e264–9. https://doiorg.publicaciones.saludcastillayleon.es/10.3399/bjgp15X684433.
Roach P, Klindukhova O, Saha C, Hudson B, Cantrell M, Marrero D. Project RedCar: Cardiovascular Disease Risk Communication for People With Type 2 Diabetes Combining the Power of Electronic Health Records and Computer-Based Multimedia Technology. Diabetes Spectrum. 2010;23(3):155–60. https://doiorg.publicaciones.saludcastillayleon.es/10.2337/diaspect.23.3.155.
Rosal MC, Ockene JK, Luckmann R, Zapka J, Goins KV, Saperia G, et al. Coronary heart disease multiple risk factor reduction. Providers’ perspectives Am J Prev Med. 2004;27(2 Suppl):54–60. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.amepre.2004.04.020.
Salmasi S, Kwan L, MacGillivray J, Bansback N, De Vera MA, Barry AR, et al. Assessment of atrial fibrillation patients’ education needs from patient and clinician perspectives: A qualitative descriptive study. Thromb Res. 2019;173:109–16. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.thromres.2018.11.015.
Tawfik MY, Mohamed RA. The impact of communicating cardiovascular risk in type 2 diabetics on patient risk perception, diabetes self-care, glycosylated hemoglobin, and cardiovascular risk. J Public Health. 2016;24(2):153–64. https://doiorg.publicaciones.saludcastillayleon.es/10.1007/s10389-016-0710-2.
van Steenkiste B, van der Weijden T, Stoffers HE, Grol R. Barriers to implementing cardiovascular risk tables in routine general practice. Scand J Prim Health Care. 2004;22(1):32–7. https://doiorg.publicaciones.saludcastillayleon.es/10.1080/02813430310004489.
Welschen LM, Bot SD, Kostense PJ, Dekker JM, Timmermans DR, van der Weijden T, et al. Effects of cardiovascular disease risk communication for patients with type 2 diabetes on risk perception in a randomized controlled trial: the @RISK study. Diabetes Care. 2012;35(12):2485–92. https://doiorg.publicaciones.saludcastillayleon.es/10.2337/dc11-2130.
Williams PA, Prabandari YS, Burfeind C, Lefebvre RC, LaBresh KA. Impact of a Pilot Intervention to Increase Physician-Patient Communication About Stroke Risk in Indonesia. Health Commun. 2016;31(12):1573–8. https://doiorg.publicaciones.saludcastillayleon.es/10.1080/10410236.2015.1082456.
Denig P, Schuling J, Haaijer-Ruskamp F, Voorham J. Effects of a patient oriented decision aid for prioritising treatment goals in diabetes: pragmatic randomised controlled trial. BMJ. 2014;349: g5651. https://doiorg.publicaciones.saludcastillayleon.es/10.1136/bmj.g5651.
Huang ES, Nathan AG, Cooper JM, Lee SM, Shin N, John PM, et al. Impact and Feasibility of Personalized Decision Support for Older Patients with Diabetes: A Pilot Randomized Trial. Med Decis Making. 2017;37(5):611–7. https://doiorg.publicaciones.saludcastillayleon.es/10.1177/0272989X16654142.
Ledford CJ. Contending mediated risk messages: a grounded theory of the physician-patient discussion of a prescription medication’s changing risk. Patient Educ Couns. 2011;83(1):14–21. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.pec.2010.04.041.
Lyles CR, Grothaus L, Reid RJ, Sarkar U, Ralston JD. Communication about diabetes risk factors during between-visit encounters. Am J Manag Care. 2012;18(12):807–15.
Ritholz MD, MacNeil T, Weinger K. Difficult conversations: adults with diabetes and the discussion of microvascular complications. Diabet Med. 2017;34(10):1447–55. https://doiorg.publicaciones.saludcastillayleon.es/10.1111/dme.13419.
Rouyard T, Leal J, Baskerville R, Velardo C, Salvi D, Gray A. Nudging people with Type 2 diabetes towards better self-management through personalized risk communication: A pilot randomized controlled trial in primary care. Endocrinol Diabetes Metab. 2018;1(3): e00022. https://doiorg.publicaciones.saludcastillayleon.es/10.1002/edm2.22.
Yao M, Zhang DY, Fan JT, Lin K, Haroon S, Jackson D, et al. The experiences of people with type 2 diabetes in communicating with general practitioners in China - a primary care focus group study. BMC Prim Care. 2022;23(1):24. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12875-022-01632-y.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57. https://doiorg.publicaciones.saludcastillayleon.es/10.1093/intqhc/mzm042.
Barker TH, Stone JC, Sears K, Klugar M, Tufanaru C, Leonardi-Bee J, et al. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid Synth. 2023;21(3):494–506. https://doiorg.publicaciones.saludcastillayleon.es/10.11124/jbies-22-00430
Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. 4. Systematic reviews of effectiveness. JBI Manual for Evidence Synthesis. Available from: https://synthesismanual.jbi.global
Moola S, Munn Z, Sears K, Sfetcu R, Currie M, Lisy K, et al. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. Int J Evid Based Healthc. 2015;13(3):163–9. https://doiorg.publicaciones.saludcastillayleon.es/10.1097/XEB.0000000000000064.
Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ Inf. 2018;34(4):285–91. https://doiorg.publicaciones.saludcastillayleon.es/10.3233/EFI-180221.
Avery L, Flynn D, van Wersch A, Sniehotta FF, Trenell MI. Changing physical activity behavior in type 2 diabetes: a systematic review and meta-analysis of behavioral interventions. Diabetes Care. 2012;35(12):2681–9. https://doiorg.publicaciones.saludcastillayleon.es/10.2337/dc11-2452.
Chew BH, Shariff-Ghazali S, Fernandez A. Psychological aspects of diabetes care: Effecting behavioral change in patients. World J Diabetes. 2014;5(6):796–808. https://doiorg.publicaciones.saludcastillayleon.es/10.4239/wjd.v5.i6.796.
Kubzansky LD, Huffman JC, Boehm JK, Hernandez R, Kim ES, Koga HK, et al. Positive Psychological Well-Being and Cardiovascular Disease: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(12):1382–96. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.jacc.2018.07.042.
Laddu D, Ma J, Kaar J, Ozemek C, Durant RW, Campbell T, et al. Health Behavior Change Programs in Primary Care and Community Practices for Cardiovascular Disease Prevention and Risk Factor Management Among Midlife and Older Adults: A Scientific Statement From the American Heart Association. Circulation. 2021;144(24):e533–49. https://doiorg.publicaciones.saludcastillayleon.es/10.1161/CIR.0000000000001026.
Lewis CC, Klasnja P, Powell BJ, Lyon AR, Tuzzio L, Jones S, et al. From Classification to Causality: Advancing Understanding of Mechanisms of Change in Implementation Science. Front Public Health. 2018;6:136. https://doiorg.publicaciones.saludcastillayleon.es/10.3389/fpubh.2018.00136.
Mazzucca S, Arredondo EM, Hoelscher DM, Haire-Joshu D, Tabak RG, Kumanyika SK, et al. Expanding Implementation Research to Prevent Chronic Diseases in Community Settings. Annu Rev Public Health. 2021;42:135–58. https://doiorg.publicaciones.saludcastillayleon.es/10.1146/annurev-publhealth-090419-102547.
Miller CJ, Barnett ML, Baumann AA, Gutner CA, Wiltsey-Stirman S. The FRAME-IS: a framework for documenting modifications to implementation strategies in healthcare. Implement Sci. 2021;16(1):36. https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s13012-021-01105-3.
Davis K, Schoenbaum SC, Audet AM. A 2020 vision of patient-centered primary care. J Gen Intern Med. 2005;20(10):953–7. https://doiorg.publicaciones.saludcastillayleon.es/10.1111/j.1525-1497.2005.0178.x.
Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–7. https://doiorg.publicaciones.saludcastillayleon.es/10.1007/s11606-012-2077-6.
Epstein RM, Fiscella K, Lesser CS, Stange KC. Why the nation needs a policy push on patient-centered health care. Health Aff (Millwood). 2010;29(8):1489–95. https://doiorg.publicaciones.saludcastillayleon.es/10.1377/hlthaff.2009.0888.
Lown BA, Clark WD, Hanson JL. Mutual influence in shared decision making: a collaborative study of patients and physicians. Health Expect. 2009;12(2):160–74. https://doiorg.publicaciones.saludcastillayleon.es/10.1111/j.1369-7625.2008.00525.x.
Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease. Circulation. 2015;132(9):873–98. https://doiorg.publicaciones.saludcastillayleon.es/10.1161/CIR.0000000000000228.
Hill-Briggs F, Adler NE, Berkowitz SA, Chin MH, Gary-Webb TL, Navas-Acien A, et al. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care. 2020;44(1):258–79. https://doiorg.publicaciones.saludcastillayleon.es/10.2337/dci20-0053.
Powell-Wiley TM, Baumer Y, Baah FO, Baez AS, Farmer N, Mahlobo CT, et al. Social Determinants of Cardiovascular Disease. Circ Res. 2022;130(5):782–99. https://doiorg.publicaciones.saludcastillayleon.es/10.1161/CIRCRESAHA.121.319811.
Walker RJ, Strom Williams J, Egede LE. Influence of Race, Ethnicity and Social Determinants of Health on Diabetes Outcomes. Am J Med Sci. 2016;351(4):366–73. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/j.amjms.2016.01.008.
Stuart-Shor EM, Berra KA, Kamau MW, Kumanyika SK. Behavioral strategies for cardiovascular risk reduction in diverse and underserved racial/ethnic groups. Circulation. 2012;125(1):171–84. https://doiorg.publicaciones.saludcastillayleon.es/10.1161/CIRCULATIONAHA.110.96849.
Hassan S, Gujral UP, Quarells RC, Rhodes EC, Shah MK, Obi J, et al. Disparities in diabetes prevalence and management by race and ethnicity in the USA: defining a path forward. Lancet Diabetes Endocrinol. 2023;11(7):509–24. https://doiorg.publicaciones.saludcastillayleon.es/10.1016/S2213-8587(23)00129-8.
Saenz C, Salinas M, Rothman RL, White RO. Personalized Lifestyle Modifications for Improved Metabolic Health: The Role of Cultural Sensitivity and Health Communication in Type 2 Diabetes Management. Journal of the American Nutrition Association. 2024:1–14. https://doiorg.publicaciones.saludcastillayleon.es/10.1080/27697061.2024.2413368
Gidlow CJ, Ellis NJ, Cowap L, Riley V, Crone D, Cottrell E, et al. Cardiovascular disease risk communication in NHS Health Checks using QRISK(R)2 and JBS3 risk calculators: the RICO qualitative and quantitative study. Health Technol Assess. 2021;25(50):1–124. https://doiorg.publicaciones.saludcastillayleon.es/10.3310/hta25500.
Kruse RL, Olsberg JE, Shigaki CL, Parker Oliver DR, Vetter-Smith MJ, Day TM, et al. Communication during patient-provider encounters regarding diabetes self-management. Fam Med. 2013;45(7):475–83.
Acknowledgements
Not Applicable.
Funding
This research study is kindly supported and generously funded by the Ng Teng Fong Foundation (Grant Reference: NTF_SRP_P1) and National Healthcare Group as part of the Personalised Cardiometabolic Risk Management program (Predict to Prevent, ‘P2P study’). The P2P study is a population health program that aims to monitor, predict, and delay the risk of macrovascular complications through early risk identification and stratification amongst patients and population groups at high risk of developing cardiovascular disease.
The funding agencies were not involved in the design, planning, screening, analysis or interpretation of the findings of this study, as well as the preparation of this manuscript in any way.
Author information
Authors and Affiliations
Contributions
Study design and conceptualisation: AC, WT; Database search and extraction of articles: YM; Screening and review of articles: AC, WT; Data extraction: AC, WT; Study quality appraisal: AC, WT, SA; Drafting and preparation of manuscript: AC; Review of manuscript: AC, WT, SA, RD. All authors approve of the final version of this manuscript.
Authors’ Twitter handles
Twitter handles: @Winnie_LL_Teo (Winnie Li-Lian Teo)
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chia, A.WY., Teo, W.LL., Acharyya, S. et al. Patient-physician communication of health and risk information in the management of cardiovascular diseases and diabetes: a systematic scoping review. BMC Med 23, 96 (2025). https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12916-025-03873-x
Received:
Accepted:
Published:
DOI: https://doiorg.publicaciones.saludcastillayleon.es/10.1186/s12916-025-03873-x